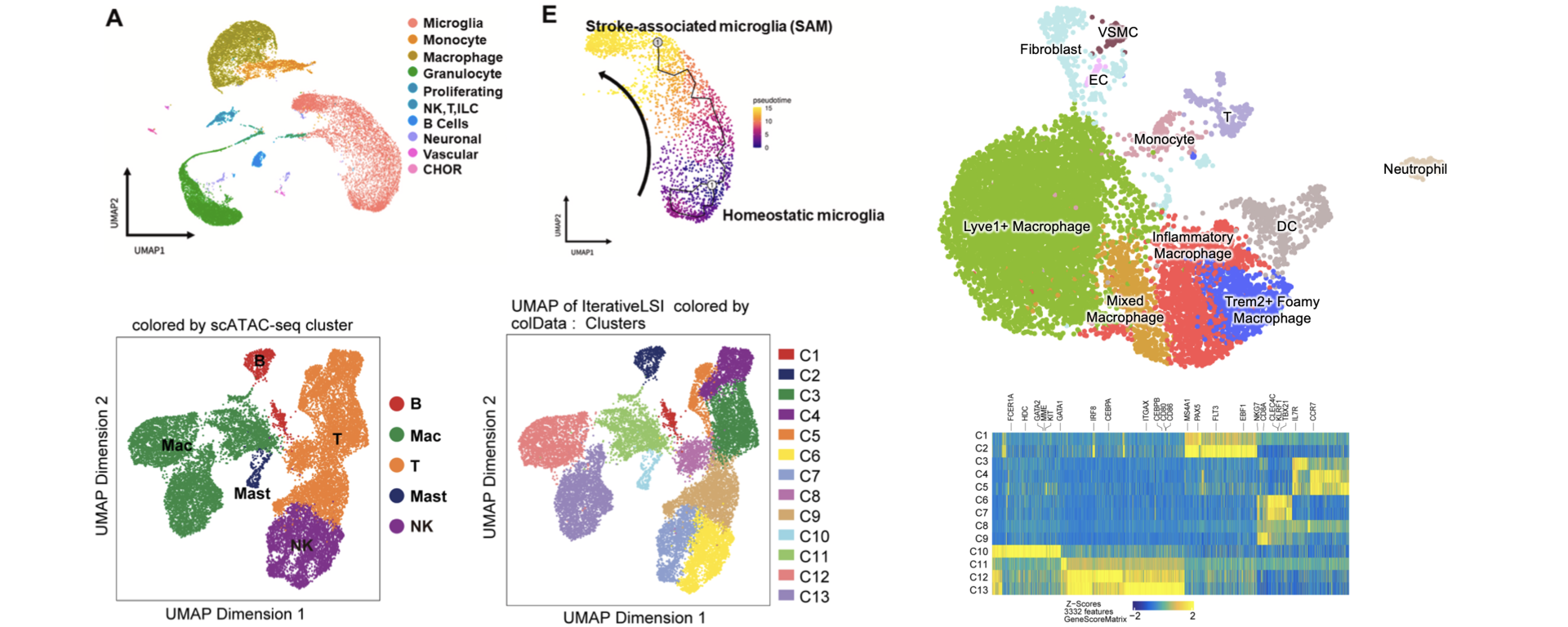
We study the immunologic aspects of a very broad range of human diseases, encompassing investigations in the fields of autoimmune diseases, inflammatory diseases and hematopoiesis such as rheumatoid arthritis, systemic lupus erythematosus, atherosclerosis and leukemia. We are interested in how cytokines and inflammatory factors regulate the activation and function of innate immune and stromal cells, with a focus on macrophages, monocytes and fibroblasts. We are currently studying how cytokines such as IFNs and TNF reprogram macrophage responses to the environment by altering transcription factor networks, chromatin states, cell metabolism, and the epigenomic landscape of enhancers. To understand of underlying mechanisms, we integrate basic science investigation of signaling and epigenetic mechanisms with translational research using disease models and analysis of human disease samples with genome-wide approaches such as single cell RNA-seq, ChIP-seq, and ATAC-seq. Our long-term research goals are to identify signaling and epigenetic mechanisms that can be targeted by new therapies for inflammatory diseases, including biological approaches to enhancing clinical outcomes.
Topics from a biological point of view:
Cytokine regulation, Plasticity in Macrophages, Epigenomics
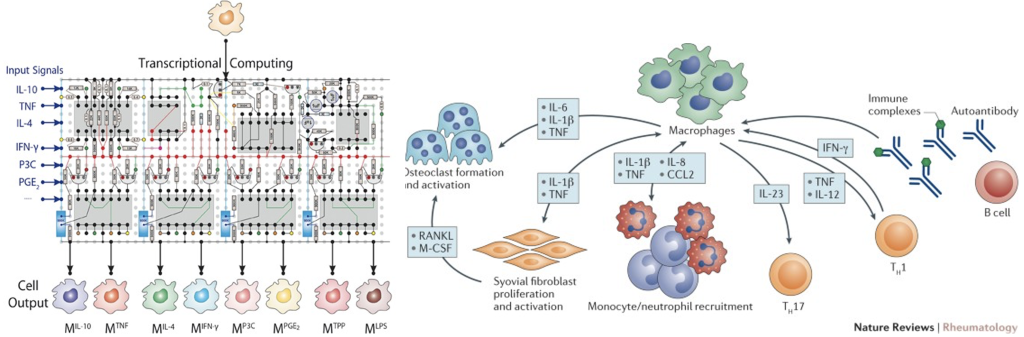
We are studying the role of macrophages/monocytes in the maintenance of homeostasis and in physiological inflammation. In particular, we are studying mechanisms of innate immune memory and cytokine cross-regulation.
Biology of Inflammation and physiological functions of inflammatory response. The research activity in our laboratory is focused on understanding the molecular control of inflammatory gene expression and on the epigenetic mechanisms linking chronic inflammation to human diseases.

Principles of the transcriptional response and epigenetic regulation. A major interest of the lab is to clarify the molecular mechanisms controlling macrophage gene expression and in particular the inflammatory gene expression program. Our laboratory is mapping the regulatory circuitry that controls cell activation state and differentiation in mice and humans.
Cellular heterogeneity. The main objective of the research activity in this unit is to understand how macrophage identity, functional specialization, and plasticity are controlled by their specialized genomic organization, enforced by specific transcription factors, and modulated by the microenvironment.
Topics from an experimental technique point of view:
Bioinformatics and NGS
Our approach involves computational integration of different data layers to understand the joint influences of interacting biological processes to comprehend how distinct regulatory layers contribute to complex phenotypes.
NGS Approach: single cell RNAseq, sc-ATACseq, ChIPseq, Pro-seq, DRIP-seq, TT-seq etc
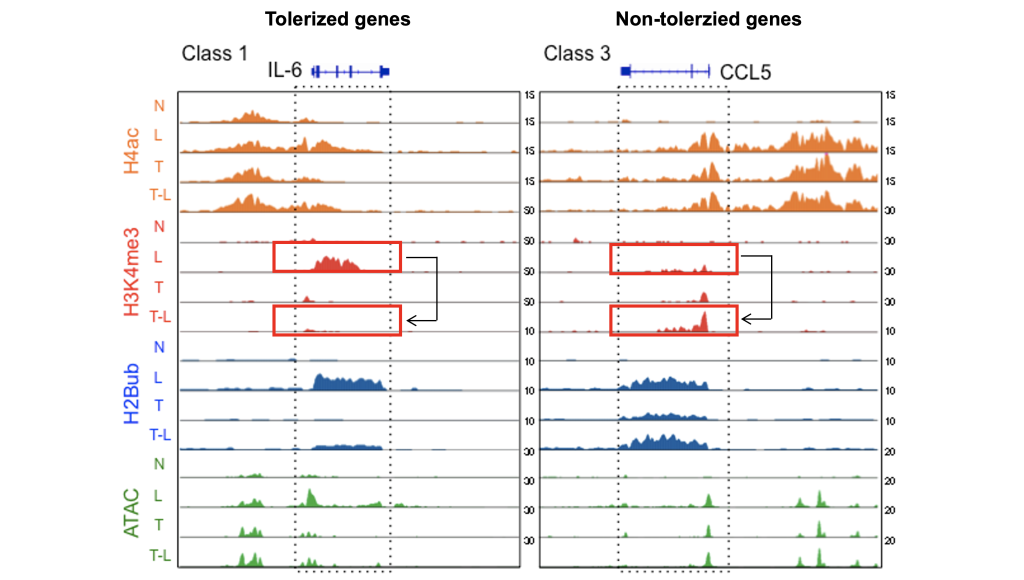
Experimental model: Human Blood, Mouse, Patient samples
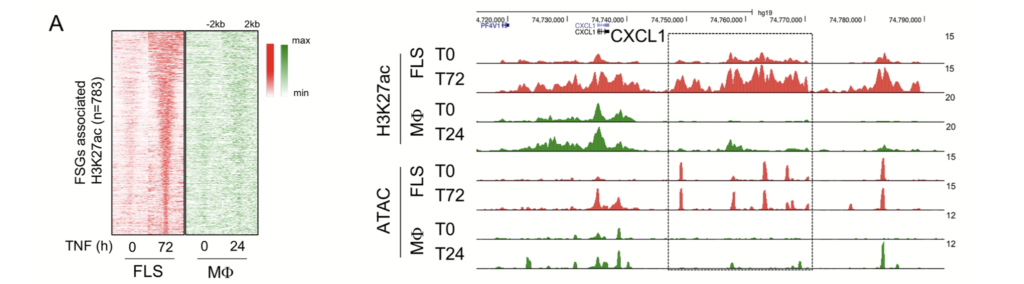
Topics from a clinical point of view:
Chronic inflammation, Autoimmune Diseases, and Leukemia
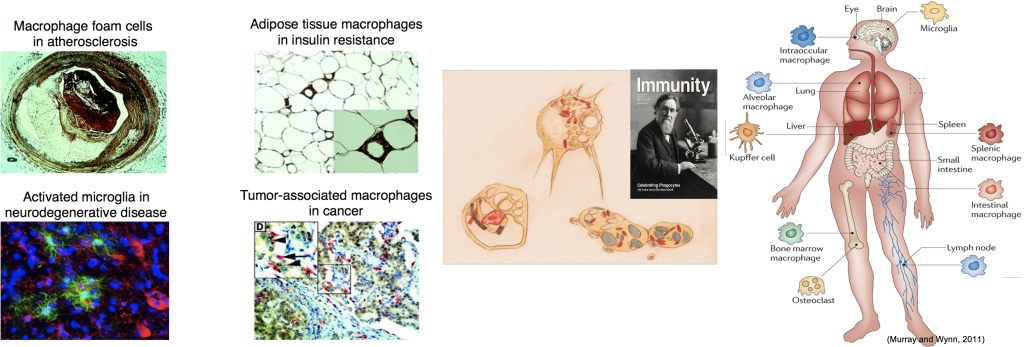
We study the immunologic aspects of a very broad range of human diseases, encompassing investigations in the fields of autoimmune diseases including rheumatoid arthritis and systemic lupus erythematosus, and leukemia (CML, MDS).
Mechanisms of autoimmunity. Mechanisms of dysregulation of immune responses leading to autoimmune diseases.
Cell fate decisions (Hematopoiesis). Our lab studies how the hematopoietic system utilizes these sets of rules the regulatory code to differentiate cells into various professional immune cells that make up the immune system.